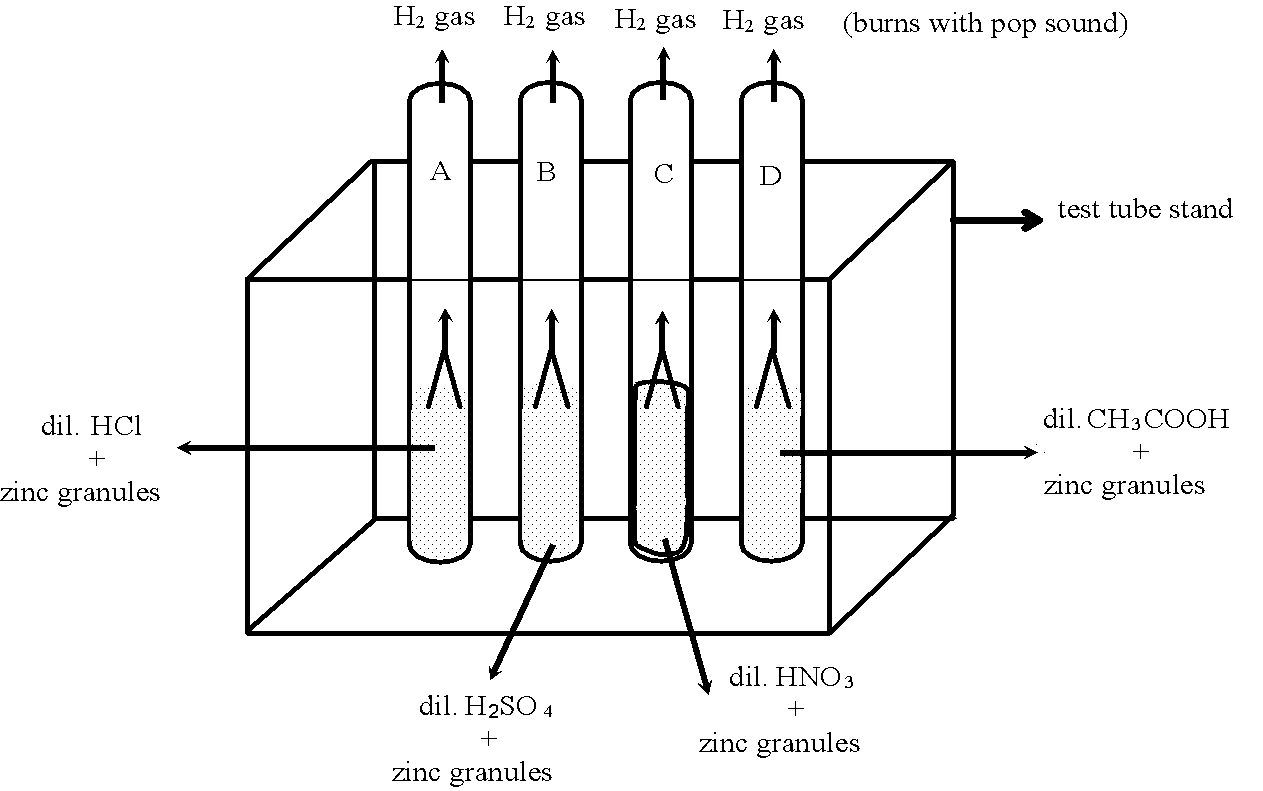
Why do all Acids Have in Common
⇒ To see what is common in all acids, let us perform the following experiment with different acids:
Experiment to illustrate chemical nature of acids or what do all acids have in common:
Take four test tubes and label them as A, B, C and D. Place them in a test tube stand. Take about 2 ml of each
dil HCl, dil ![]() , dil
, dil ![]() and dil CH3COOH in test tubes
A, B, C and D respectively. Now add few pieces of zinc granules in each test tube.
and dil CH3COOH in test tubes
A, B, C and D respectively. Now add few pieces of zinc granules in each test tube.
|
Figure-Reaction of Zn metal with different acids |
Observation: There is evolution of hydrogen gas (H2)in each test tube which burns with a pop sound on bringing a burning candle near the mouth of tubes.
Conclusion: Hydrogen is common in all acids i.e. all acids contain hydrogen which they liberate when they react with active metals.
Thus we can say that acids are the substances which contain hydrogen ion, which they liberate when they react with active metals.
All acids contain hydrogen but all hydrogen containing compounds are not acids, for example, glucose (C6H12O6) and alcohol (C2H5OH) contain hydrogen but they are not acids.
It can be explained more clearly by following experiment.
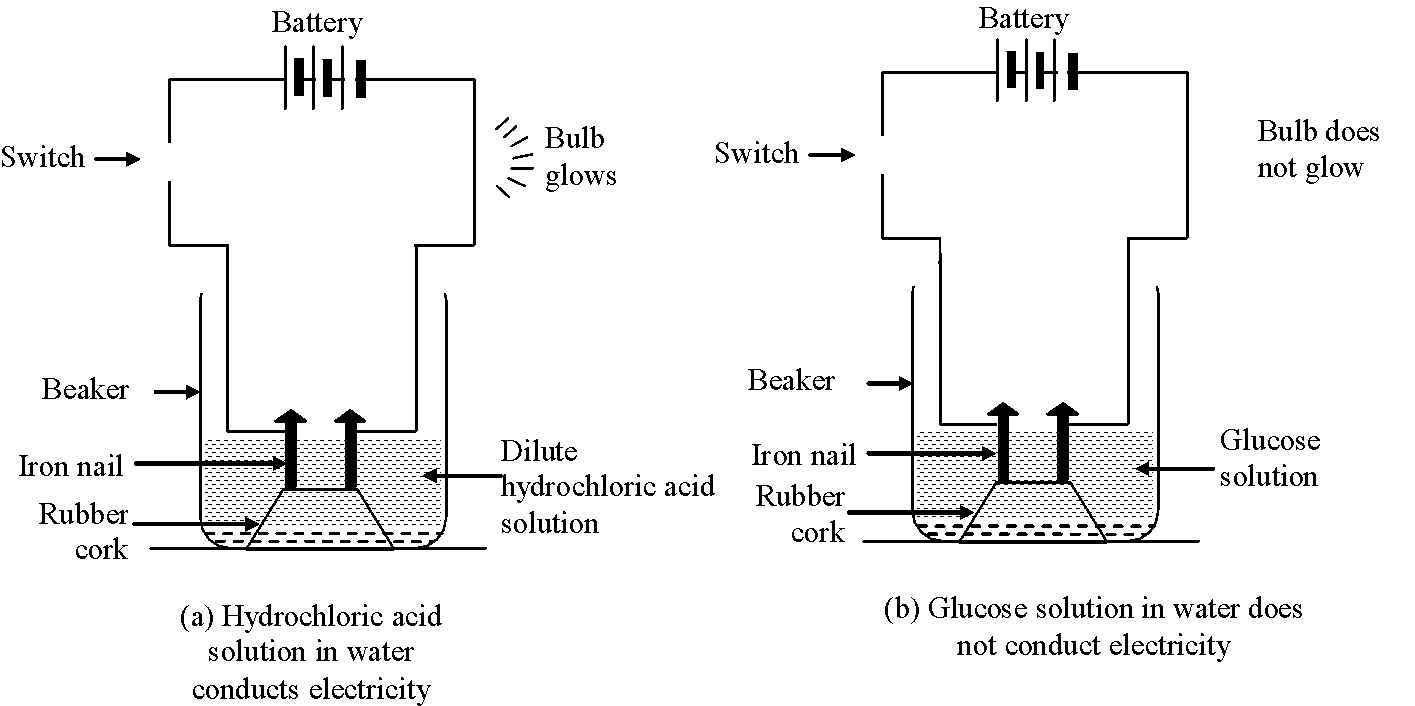
Experiment to show that all compounds containing hydrogen are not acids: The experiment is based on the fact that acids conduct electricity through their aqueous solutions.
-
Take aqueous solutions of hydrogen containing compounds like hydrochloric acid (HCl), sulphuric acid
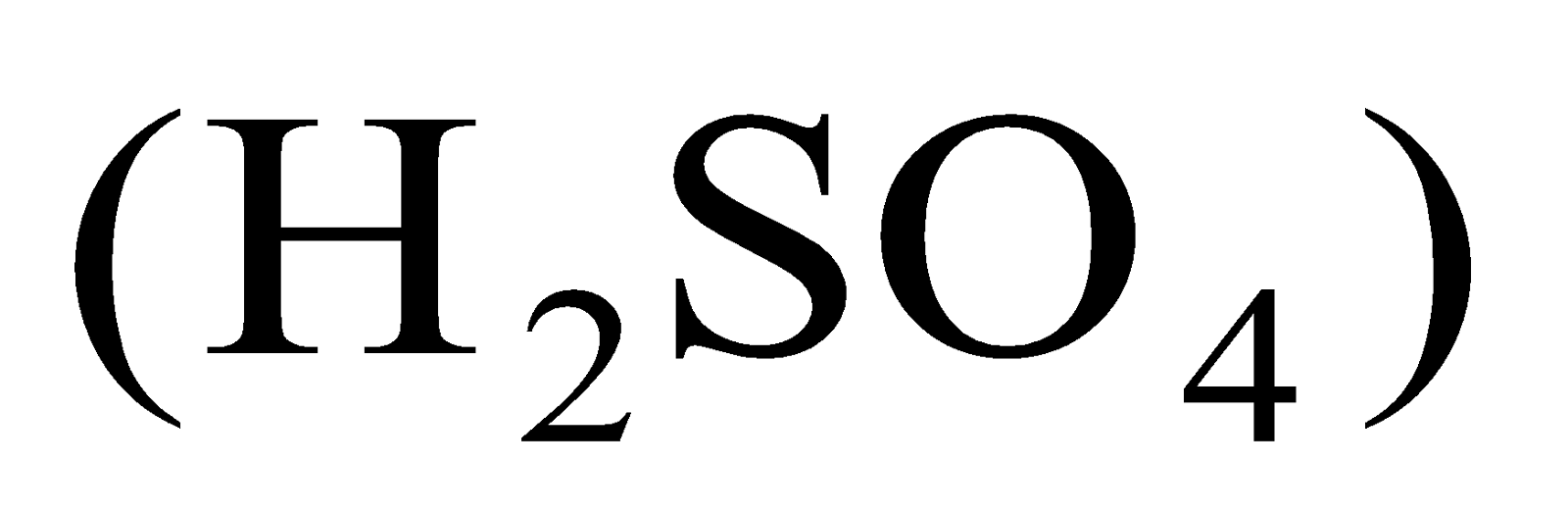 , glucose
, glucose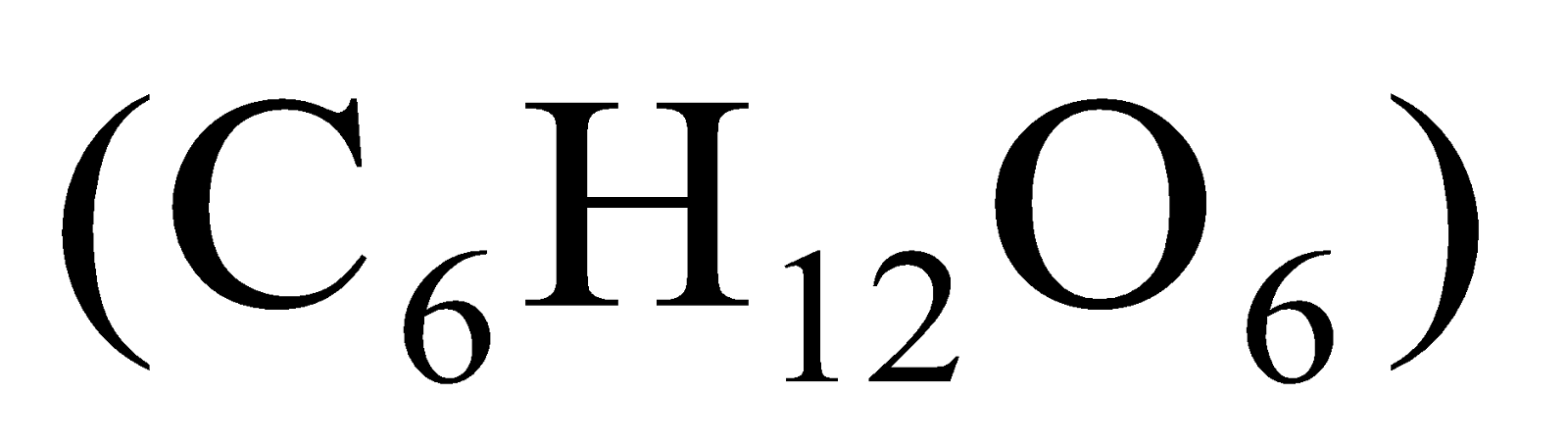 and alcohol
and alcohol  in 4 beakers respectively.
in 4 beakers respectively. - Fix two iron nails on the rubber cork and place the cork in each beaker
- Connect the nails to the two terminals of a 6 volt battery through a switch and a bulb in each beaker as shown in the following figures.
- Switch on the current in each case
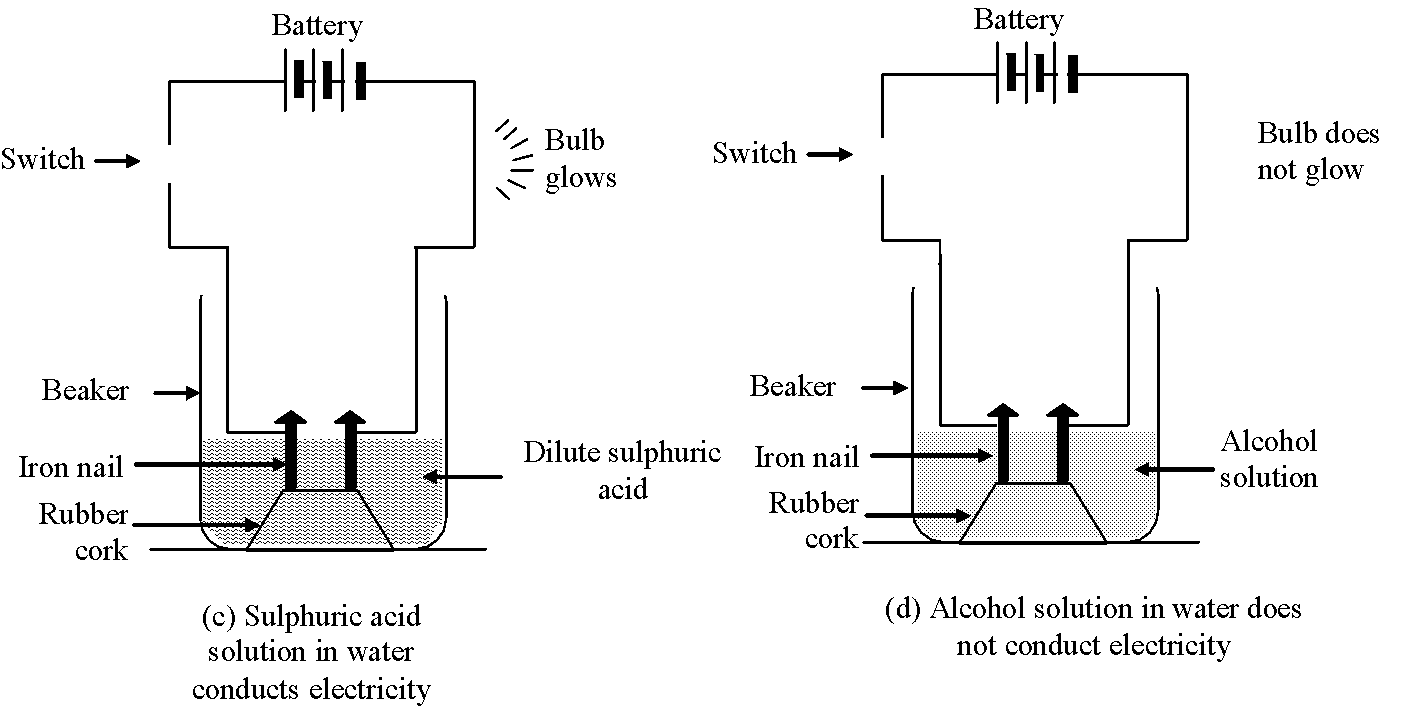
Observation:
(i) Bulb starts glowing in arrangements (a) and (c) containing aqueous solutions of HCl and ![]() acids respectively. It shows aqueous solutions of
hydrochloric acid (HCl) and sulphuric acid
acids respectively. It shows aqueous solutions of
hydrochloric acid (HCl) and sulphuric acid ![]() conduct electricity.
conduct electricity.
(ii) Aqueous solutions of glucose ![]() and alcohol
and alcohol ![]() do not conduct electricity (i.e. they do not allow
electricity to pass through them) as bulb does not glow in arrangements b and d containing aqueous solutions of
glucose and alcohol.
do not conduct electricity (i.e. they do not allow
electricity to pass through them) as bulb does not glow in arrangements b and d containing aqueous solutions of
glucose and alcohol.
Explanation: Conduction of electricity through the aqueous
solutions of acids (HCl and H2SO4) is due to the ions present in them. For example, aqueous solution of ![]() contains
contains ![]() and
and ![]() ions. These ions can carry electric
current and thus are responsible for conduction of electricity through HCl and
ions. These ions can carry electric
current and thus are responsible for conduction of electricity through HCl and ![]() solutions. On the other hand aqueous solutions of
glucose and alcohol (hydrogen containing compounds) do not contain
solutions. On the other hand aqueous solutions of
glucose and alcohol (hydrogen containing compounds) do not contain ![]() ions or any other ions. Due to absence of ions,
aqueous solutions of glucose and alcohol do not conduct electricity.
ions or any other ions. Due to absence of ions,
aqueous solutions of glucose and alcohol do not conduct electricity.
Conclusion: From the above experiment, we lead to a conclusion
that only those hydrogen containing compounds are acidic which when dissolved in water give ![]() ions in the solution. Thus the definition of acid
is modified as:
ions in the solution. Thus the definition of acid
is modified as:
Acids are the substances which contain hydrogen and which when dissolved in water give ![]() ions in the solution. This is called Arrhenius
definition of acids given by Arrhenius in 1884.
ions in the solution. This is called Arrhenius
definition of acids given by Arrhenius in 1884.
WHAT HAPPENS TO AN ACID IN WATER SOLUTION?
It is observed that acidic behaviour of acids is due to the presence of H+ ions in them, which they give only in presence of water. So in
the absence of water, a substance will not form ![]() ions and hence will not show its acidic behavior.
It can be explained more clearly by following experiments:
ions and hence will not show its acidic behavior.
It can be explained more clearly by following experiments:
Experiment: Take about 1–2 g of NaCl in a dry test tube. Add
some concentrated ![]() into the test tube. Following reaction takes place
producing hydrogen chloride (HCl) gas
into the test tube. Following reaction takes place
producing hydrogen chloride (HCl) gas
![]()
sodium chloride sulphuric acid sodium hydrogen sulphate hydrogen chloride gas (conc.)
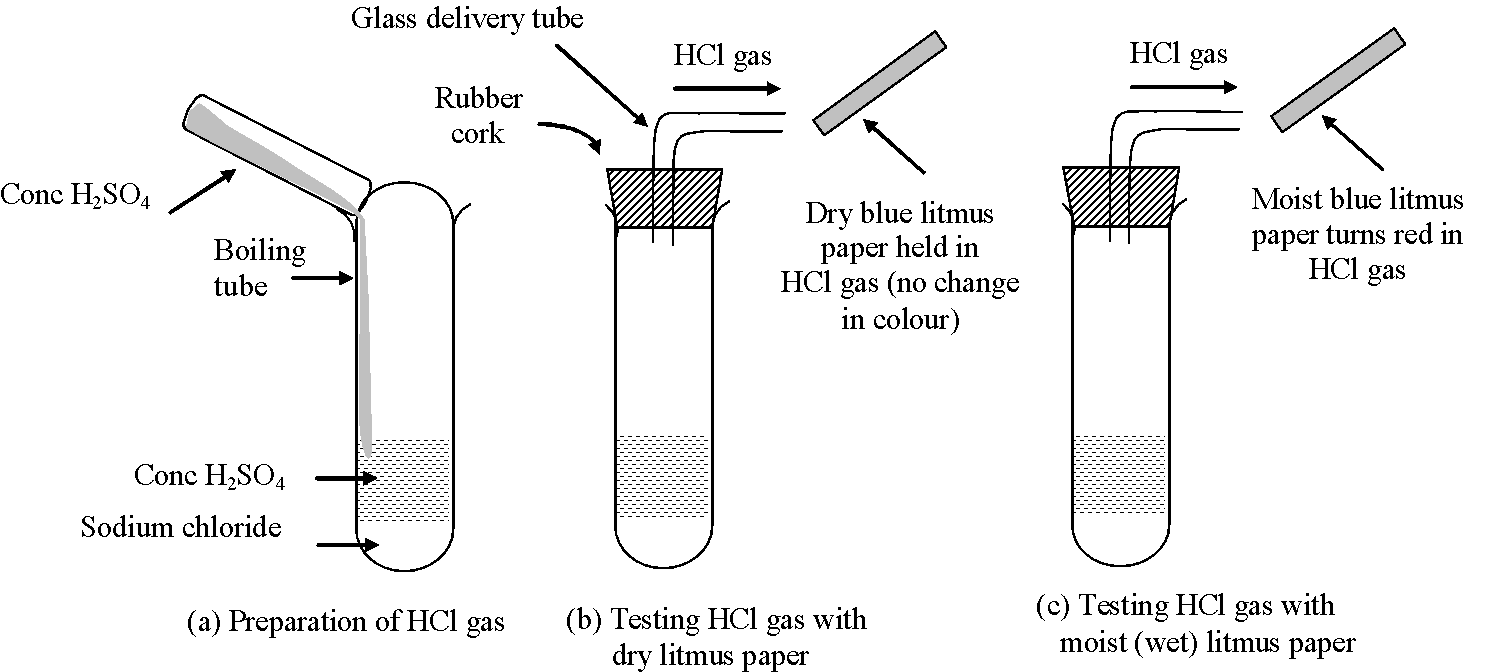
Now bring a dry blue litmus paper and a wet (or moist) blue litmus paper near the mouth of test tube (which contains HCl gas)
Observation:
(i) The dry litmus paper does not turn red. It shows that HCl gas does not behave as an acid in absence of water (since there is no water in dry litmus paper).
(ii) The wet (or moist) litmus paper turns red. It shows that HCl gas acts as an acid only in presence of water (which is present in moist or wet litmus paper).
Explanation:
(i) When HCl gas come in contact with dry litmus paper, then HCl does not dissociate into ions (i.e ![]() and
and ![]() ions) due to absence of water in dry litmus paper.
ions) due to absence of water in dry litmus paper.
Since ![]() ions are responsible for acidic behaviour of acids,
HCl gas does not show acidic behaviour with dry litmus paper and thus it does not turn the blue litmus red (due
to absence of H+ ion in dry HCl gas).
ions are responsible for acidic behaviour of acids,
HCl gas does not show acidic behaviour with dry litmus paper and thus it does not turn the blue litmus red (due
to absence of H+ ion in dry HCl gas).
![]() Dissociation does not occur.
Dissociation does not occur.
(ii) When HCl gas comes in contact with wet litmus paper, then HCl dissociates into ![]() and
and ![]() ions due to dissociation of HCl in water present in
wet litmus paper.
ions due to dissociation of HCl in water present in
wet litmus paper.
Since ![]() ions are responsible for acidic behaviour of acids,
HCl gas shows acidic behaviour with wet litmus paper and thus it turns it into red (due to presence of
ions are responsible for acidic behaviour of acids,
HCl gas shows acidic behaviour with wet litmus paper and thus it turns it into red (due to presence of ![]() ions in wet HCl gas) i.e.
ions in wet HCl gas) i.e.
![]()
(acts as acid) (dissociation occurs)
Such dissociation of a covalent molecule like HCl into ions in the presence of water is called ionization. The ionization of HCl is shown more clearly as follows:
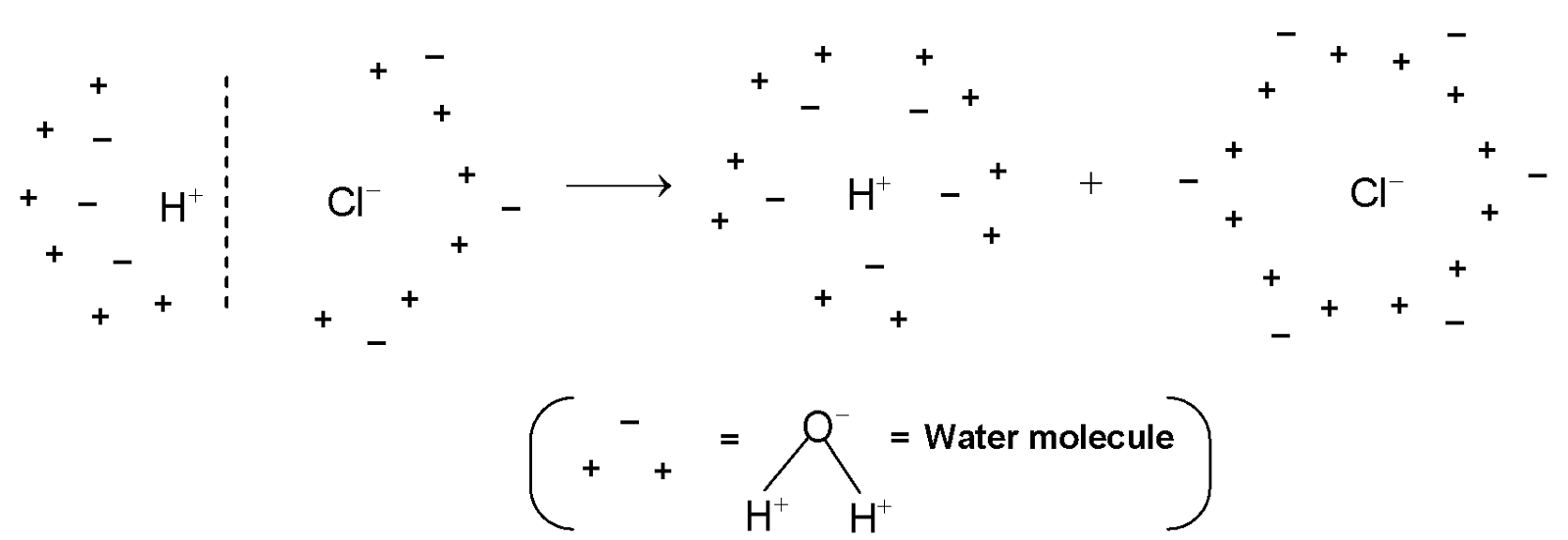
Dissociation of HCl into H+ and Cl− ions in presence of water.
It is clear from the figure that, after dissociation of HCl, a number of water molecules remain attached to
![]() . Hence they are represented as H+(aq) and Cl–(aq) (aq indicating water molecules)
. Hence they are represented as H+(aq) and Cl–(aq) (aq indicating water molecules)
Alternatively, ![]() ions combine with water molecule to form an ion
called hydronium ion.
ions combine with water molecule to form an ion
called hydronium ion.
![]()
hydrogen ion water molecule hydronium ion
Thus ![]() does not exist freely in water, but exist in
combination with water molecules. Hence, we represent it as
does not exist freely in water, but exist in
combination with water molecules. Hence, we represent it as ![]() .
.
Conclusion: The properties of an acid is due to ![]() ions or hydronium ions
ions or hydronium ions ![]() which it gives in the aqueous solution.
which it gives in the aqueous solution.
or
Acidic properties of acids are due to presence of ![]() ions or
ions or ![]() ions which they produce only in presence
of water.
ions which they produce only in presence
of water.
or
In absence of water, a substance will not form ![]() ions or
ions or ![]() ions and hence will not show its acidic
behaviour.
ions and hence will not show its acidic
behaviour.
Greater the amount of H+(aq) ions in the solution, stronger is the acid .