Some Important Chemical Compound
Acid Base And Salt of Class 10
SODIUM CHLORIDE- COMMON SALT (TABLE SALT):
Sodium chloride (NaCI) also called common salt or table salt is the most essential part of our diet. Chemically it is formed by the reaction between solutions of sodium hydroxide and hydrochloric acid. Sea water is the major source of sodium chloride where it is present in dissolved form along with other soluble salts such as chlorides and sulphates of calcium and magnesium; It is separated by some suitable methods. Deposits of the salts are found in different parts of the world and are known as rock salt. When pure, it is a white crystalline solid, However, it is often brown due to the presence of impurities.
Occurrence and extraction of common salt:
The common salt occurs naturally in sea water, inland lakes and in rock salt. The extraction of common salt from these sources is given below:
Common salt from sea water
Sea water contains many dissolved salts in it. The major salt present in sea water is common salt (or NaCl). The common salt is obtained from sea water by the process of evaporation which is done as follows:
Sea water is trapped in large shallow pools and allowed to stand there. The sun’s heat evaporates the water slowly and common salt is left behind. This salt contains impurities of MgCl2, MgSO4 etc and thus purified by removing these impurities by suitable method before it is sold in the market.
Common salt from inland lakes
Large quantities of salt are obtained by natural evaporation of water of inland lakes e.g. Sambhar lake in Rajasthan (India), Great salt lake (Utah, USA) and Lake Elton (Russia).
Common salt from underground deposits
The large crystals of common salt found in underground ellipsoids is called “Rock salt”. It is usually brown due to presence of impurities in it. Rock salt is mined from underground deposits just like coal.
Common salt is an important starting material for the production of a number of other chemicals such as
- Sodium hydroxide (caustic soda).
- Calcium oxychloride (bleaching powder).
- Sodium carbonate (washing soda) .
- Sodium hydrogen carbonate (baking soda) and many others.
Uses:
(i) Essential for life: Sodium chloride is quite essential for life. Biologically, it has a number of functions to perform such as in muscle contraction, in conduction of nerve impulse inthe nervous system and is also converted in hydrochloric acid which helps in the digestion of food in the stomach. When we sweat, there is loss of sodium chloride along with water. It leads to muscle cramps. Its loss has to be compensated suitably by giving certain salt preparations to the patient. Electrol powder is an important substitute of common salt.
(ii) Raw material for chemicals: Sodium chloride is also a very useful raw material for different chemicals. A few out of these are hydrochloric acid (HCl), washing soda (Na2CO3.10H2O), baking soda (NaHCO3) etc. Upon electrolysis of a strong solution of the salt (brine), sodium hydroxide, chlorine and hydrogen are obtained. Apart from these, it is used in leather industry for the leather tanning. In severe cold, rock salt is spread on icy roads to melt ice. It is also used as a fertilizer for sugar beet.
Electrolysis of aqueous solution of NaCI:
![]()
reaction takes place in two steps
(i) 2Cl−→ CI2 (g) + 2e− (anode reaction)
(ii) 2H2O + 2e−→ H2 + OH− (cathode reaction)
WASHING SODA:
(Na2CO3.10H2O)
Chemical name: Sodium carbonate decahydrate
Chemical formula: Na2CO3. 10H2O
Sodium carbonate is recrystallised by dissolving in water to get washing soda it is a basic salt.
Na2CO3 + 10H2O → Na2CO3.10H2O
(Sodium (Washing soda)
Carbonate)
Manufacture of washing soda
Washing soda is manufactured from sodium chloride (or common salt) in the following three steps:
Manufacture of sodium hydrogen carbonate (baking soda) by solvay process: A cold and concentrated solution of
sodium chloride (brine) is reacted with ammonia and ![]() to obtain sodium hydrogen carbonate
to obtain sodium hydrogen carbonate
NaCl + H2O + NH3 + CO2 → NaHCO3 + NH4Cl
sodium water ammonia carbondioxide sodium ammoniumchloride
chloride bicarbonate
Thermal decomposition of sodium hydrogen carbonate (or baking soda): On heating sodium hydrogen carbonate decomposes to form sodium carbonate.
![]()
sod. hydrogen sod. carbonate carbon dioxide water
carbonate (Anhydrous)
Re-crystallization of sodium carbonate (or soda ash):
Anhydrous sodium carbonate (or soda ash) obtained in step 2 is dissolved in water and subjected to re-crystallization. As a result, crystals of washing soda (sodium carbonate decahydrate) are obtained.
![]()
soda ash water washing soda
(ii) Properties of Washing Soda
(a) Colour and state: It is a transparent crystalline solid (when freshly prepared) containing 10 molecules of water of crystallisation.
(b) Action of air: On exposure to air, washing soda crystals lose 9 molecules of water of crystallisation to
form a monohydrate which is a white powder ![]()
(transparent crystals)
This process is called efflorescence
(c) Action of heat: On heating, washing soda loses all the molecule of water and becomes anhydrous.
![]()
Hydrated washing soda Anhydrous sodium water
carbonate (soda ash)
Uses:
- It is used as cleansing agent for domestic purposes.
- It is used in softening hard water and controlling the pH of water.
- It is used in manufacture of glass.
- Due to its detergent properties, it is used as a constituent of several dry soap powders.
- It also finds use in photography, textile and paper industries etc.
- It is used in the manufacture of borax (Na2B4 O7.10H2O).
BAKING SODA (NaHCO3)
Baking soda is sodium hydrogen carbonate or sodium bicarbonate (NaHCO3).
(i) Manufacture of baking soda
(a) On large scale: Baking soda is produced on a large scale by reacting a cold and concentrated solution of sodium chloride (called brine) with ammonia and carbon dioxide.
![]()
sodium chloride water ammonia carbon dioxide Baking soda ammonium
chloride
This process is called solvay process.
(b) On small scale: On a small scale baking soda can be prepared in the laboratory by passing ![]() gas through aqueous sodium carbonate solution.
gas through aqueous sodium carbonate solution.
![]()
sodium carbonate water carbon dioxide baking soda
OR
![]()
(ii) Properties of Baking soda
(a) Colour and state
It is a white crystalline solid.
(b) Alkaline nature
It is mild, non-corrosive base. The alkaline nature of baking soda is due to salt hydrolysis.
![]()
baking soda water strong base weak acid
(sodium hydrogen carbonate)
![]()
![]()
![]()
Thus, salt solution is basic due to hydrolysis of ![]() ions
ions
Action of heat
When solid baking soda (or its solution) is heated it decomposes to give sodium carbonate with the evolution of
![]() gas.
gas.
![]()
baking soda soda carbonate water carbon dioxide gas
The above reaction takes places when baking soda is heated during the cooking of food. Since baking soda gives
![]() on heating, it is used as a constituent of baking
powder.
on heating, it is used as a constituent of baking
powder.
Uses:
(i) It is used in the manufacture of baking powder. Baking powder is a mixture of potassium hydrogen tartarate and sodium bicarbonate. During the preparation of bread the evolution of carbon dioxide causes bread to rise (swell).
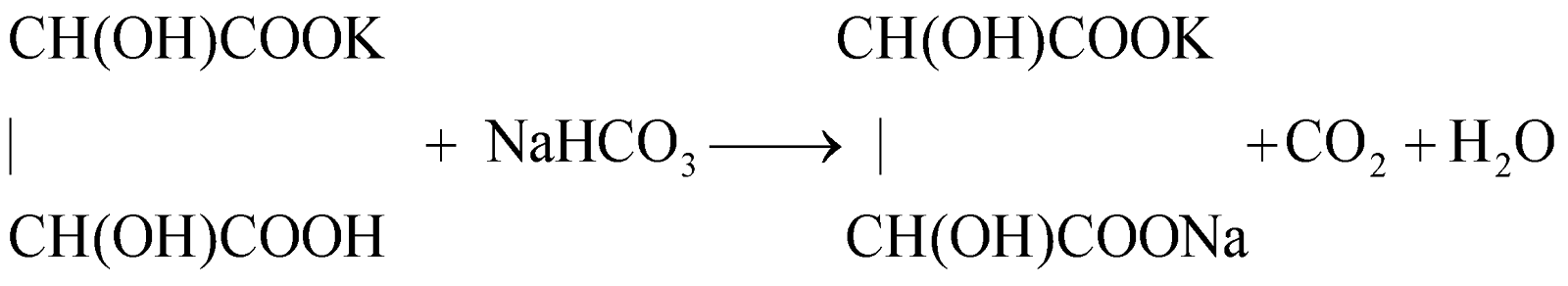
(ii) It is largely used in the treatment of acid spillage and in medicine as soda bicarb, which acts as an antacid.
(iii) It is an important chemical in the textile, tanning, paper and ceramic industries.
(iv) It is also used in a particular type of fire extinguishers. The following diagram shows a fire extinguisher that uses NaHCO3 and H2SO4 to produce CO2 gas. The extinguisher consists of a conical metallic container (a) with a nozzle (Z) at one end. A strong solution of NaHCO3 is kept in the container. A glass ampoule (P) containing H2SO4 is attached to a knob (K) and placed inside the NaHCO3 solution. The ampoule can be broken by hitting the knob. As soon as the acid comes in contact with the NaHCO3 solution, CO3 gas is formed. When enough pressure in built up inside the container, CO2 gas rushes out through the nozzle (Z). Since CO2 does not support combustion, a small fire can be put out by pointing the nozzle towards the fire. The gas is produced according to the following reaction.
2NaHCO3 (aq) + H2SO4(aq) → Na2SO4 (aq) + 2H2O(l) + 2CO2(g)
BLEACHING POWDER
(CaOCl2.4H2O, CaCl2.(OH)2. H2O)
Bleaching powder is commercially called 'chloride of lime or 'chlorinated lime'. It is principally calcium oxychloride having the following formula:
![]()
Bleaching powder is prepared by passing chlorine over slaked lime at 313 K.
Ca(OH)2(aq) + CI2 (g) ![]() Ca(OCI)CI(s) + H2O (g)
Ca(OCI)CI(s) + H2O (g)
Slaked lime Bleaching powder
|
|
Beaching powder is not a compound but a mixture of compounds: CaOCl2.4H2O, CaCI2.Ca(OH)2.H20 |
(i) Manufacture of bleaching powder
The bleaching powder is manufactured by the action of chlorine gas (produced as a bi-product during manufacture
of caustic soda) on dry slaked lime ![]() . The reactions involved are:
. The reactions involved are:
![]()
Slaked lime chlorine bleaching powder water
Uses:
- It is commonly used as a bleaching agent in paper and textile industries.
- It is also used for disinfecting water to make water free from germs.
- It is used to prepare chloroform.
- It is also used to make wool shrink-proof.
Sodium hydroxide NaOH (caustic soda)
Sodium hydroxide is commonly known as caustic soda having chemical formula NaOH. It is a strong base.
(i) Manufacture of sodium hydroxide (NaOH)
Sodium hydroxide is manufactured by the electrolysis of a concentrated aqueous solution of sodium chloride (called brine) i.e., when electricity is passed through a concentrated aqueous solution of sodium chloride (called brine), it decomposes to form sodium hydroxide, chlorine and hydrogen.
![]()
sodium chloride water sodium hydroxide chlorine hydrogen
(brine) (caustic soda)
This process is called correct-alkaliprocess because of products formed: chlor for chlorine and alkali for sodium hydroxide.
During electrolysis, ![]() gas is produced at the anode (positive electrode),
gas is produced at the anode (positive electrode),
![]() gas is produced at the cathode (negative electrode)
and NaOH solution is produced near the cathode. These products i.e.
gas is produced at the cathode (negative electrode)
and NaOH solution is produced near the cathode. These products i.e. ![]() obtained by chlor-alkali process have a
large number of uses described below one by one.
obtained by chlor-alkali process have a
large number of uses described below one by one.
(ii) Uses of sodium hydroxide (NaOH)
Sodium hydroxide (NaOH) is used
- For making soaps and detergents.
- For making artificial textile fibres.
- For making paper.
- In de-greasing metals.
- As reagent in laboratory.
- In absorbing poisonous gases.
- In petroleum industry.
Uses of chlorine (Cl2)
(a) As disinfectant and germicide for sterilization of drinking water and water in swimming pools.
(b) In manufacture of chlorofluorocarbons ![]() used as refrigerants.
used as refrigerants.
(c) In manufacture of PVC (polyvinyl chloride) used for making shoe soles.
(d) In bleaching of wood pulp and cotton fibres.
(e) In manufacture of pesticides.
Uses of hydrogen (H2)
(i) To make ammonia for fertilizers.
(ii) In metallurgy to reduce heavy metal oxide to metals.
(iii) In hydrogenation of vegetable oils to form solid fats.
(iv) Liquid hydrogen is used as a fuel for rockets.
Hydrogen and chlorine (two products of chlor-alkali process), combine to produce another important chemical called hydrochloric acid (HCl).
So we will now give some of the uses of HCl.
Uses of hydrochloric acid (HCl)
(i) For cleaning steel.
(ii) In the preparation of ammonium chloride.
(iii) In medicines and cosmetics.
(iv) In making plastics like PVC.
(v) As a reagent in the laboratory.
(vi) In making aqua regia (after mixing with HNO3) for dissolving gold and platinum. The uses of
NaOH, Cl2, ![]() can be shown more clearly in the figure.
can be shown more clearly in the figure.
|
|
Aqua-Regia is three parts conc. HCl and 1 part conc. HNO3 i.e. in Aqua-Regia HCl and HNO3 are present in 3 : 1 ratio. |
ARE THE CRYSTALLINE SALTS REALLY DRY
The crystalline salts or crystals of salts appear to be dry but actually they are not. They contain water of crystallization. It can be explained as follows:
Water of crystallization: The fixed number of water molecules present in one formula unit of salt is called water of crystallization.
For example
-
Sodium carbonate crystals (washing soda crystals) contains 10 molecules of water of crystallization in one
formula unit and hence written as
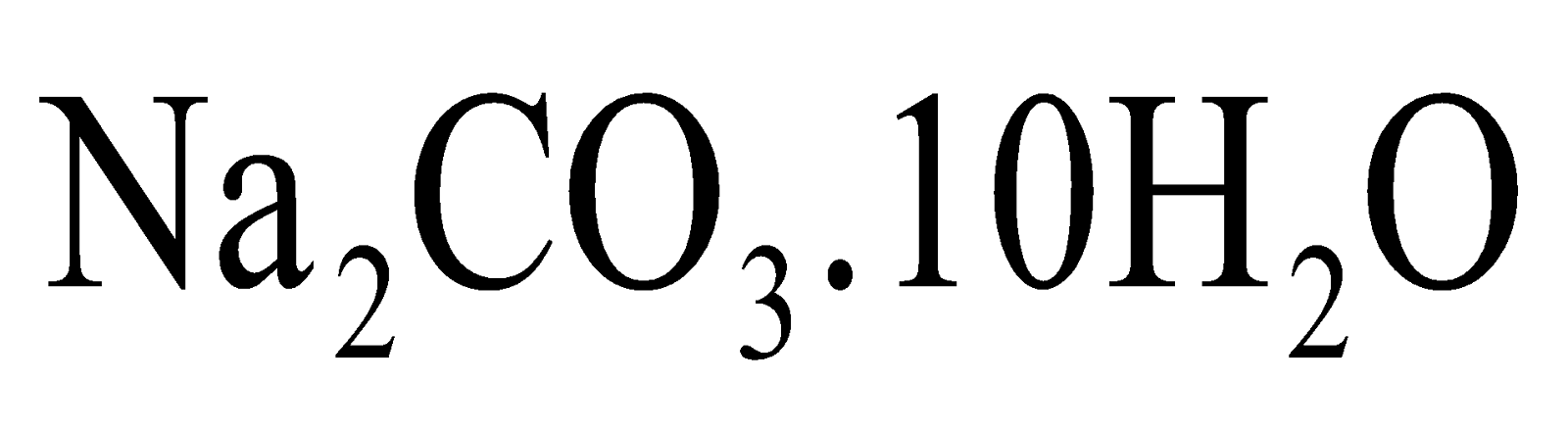 .
. -
Calcium sulphate crystals (gypsum crystals) contain 2 molecules of water of crystallization in one formula
unit and hence written as
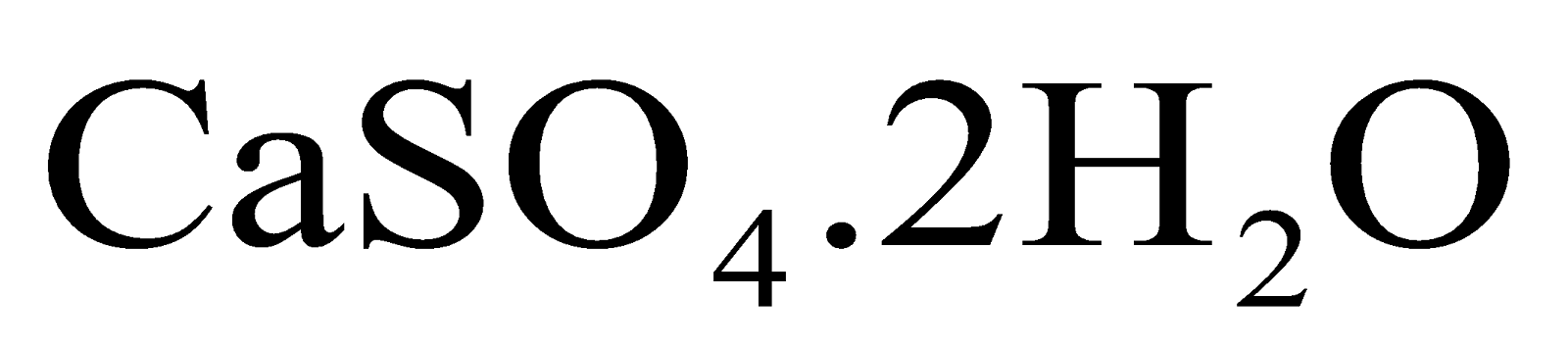 .
. -
Copper sulphate crystals contain 5 molecules of water of crystallization in one formula unit and hence
written as
 .
.
It is clear from the above examples that water of crystallization is not free water, so it does not wet the salts. Thus the crystalline salts which seem to be dry contain water of crystallization. It can be explained more clearly by following experiment.
Experiment to test the presence of water of crystallization in a crystalline salt.
Take a few crystals of copper sulphate ![]() in a dry test tube. Copper sulphate
crystals are blue in colour. Heat the test tube.
in a dry test tube. Copper sulphate
crystals are blue in colour. Heat the test tube.
Observation:
(i) Blue copper sulphate crystals turn white.
(ii) Water vapours appear on the upper parts inside the test tube.
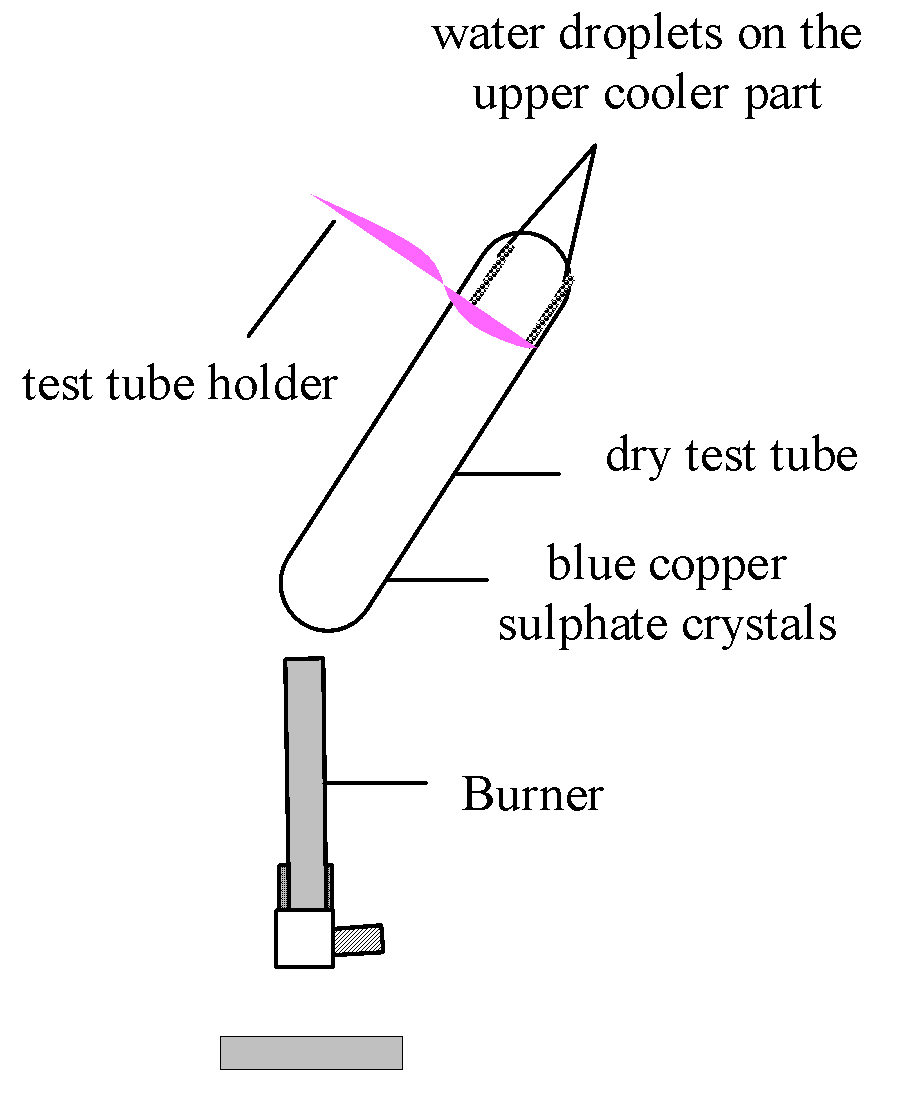
Figure-Experiment to test the presence of water of crystallisation in copper sulphate crystals
Explanation: The blue copper sulphate crystals ![]() on heating gives out water vapours which
condensed on the upper parts of the test tube and the salt left behind was anhydrous copper sulphate
on heating gives out water vapours which
condensed on the upper parts of the test tube and the salt left behind was anhydrous copper sulphate![]() which was white in colour.
which was white in colour.
Copper sulphate copper sulphate
crystals (white, anhydrous)
(blue, hydrated)
Conclusion:
Crystalline salts contain water of crystallization which are lost on heating.
Types of salts on the basis of water of crystallization
Salts are classified into two types on the basis of water of crystallization
(i) Anhydrous salts: The saltswhich contain no water of
crystallization e.g. ![]() .
.
(ii) Hydrated salts: The salts which contain a fixed number of water molecules of crystalisation. A few examples of these salts are
(a) Copper sulphate ![]() .
.
(b) Washing soda ![]() .
.
(c) Gypsum ![]() .
.
(d) Plaster of paris (CaSO4⋅½H2O).
Out of these plaster of paris is very useful salt which is discussed below:
PLASTER OF PARIS
(![]() )
)
(a) Preparation:
It is prepared by heating gypsum (CaSO4.2H2O) at about 373 K in large steel pots with mechanical stirrer, or in a revolving furnace.
or
(Calcium sulphate hemihydrate)
The temperature is carefully controlled, as at higher temperature gypsum is fully dehydrated. The properties of dehydrated gypsum are completely different from those of Plaster of Paris
Properties of plaster of Paris (POP)
(a) Colour and state: It is a white powder.
(b) Reaction with water: Setting of Plaster of Paris (or POP).
When POP is mixed with water and left for half an hour to one hour, it sets to a hard mass due to rehydration of POP to gypsum.
![]()
P.O.P. water gypsum
(c) Effect of heat: When POP is heated at 473K, it forms anhydrous calcium sulphate ![]() which is known as dead burnt plaster. It
has no setting property as it takes up water very slowly.
which is known as dead burnt plaster. It
has no setting property as it takes up water very slowly.
(P.O.P.) dead burnt plaster
Uses:
When finely powdered Plaster of Paris is mixed with water and made into a paste, it quickly sets into a hard mass. In the process, its volume also increases slightly. These properties find a number of uses. Addition of water turns Plaster of Paris back into gypsum.
- It is used in the laboratories for sealing gaps where airtight arrangement is required.
- It is also used for making toys, cosmetics and casts of statues.
- It is used as a cast for setting broken bones.
- It also finds use in making mold’s in pottery.
- It is also used for making surfaces smooth and for making designs on walls and ceilings.

