INDICATORS
Indicator indicates the nature of particular solution whether acidic, basic or neutral. Apart from this, indicator also represents the change in nature of the solution from acidic to basic and vice versa. Indicators are basically coloured organic substances extracted from different plants.
INDICATORS SHOWING DIFFERENT COLOURS IN ACIDIC AND BASIC MEDIUM
LITMUS SOLUTION:
Litmus solution is a purple coloured dye extracted from the lichen plant. It is very interesting to note that litmus solution (purple colour) itself is neither acidic nor basic. To use it as an indicator, it is made acidic or alkaline.
The alkaline form of litmus solution is blue in colour and called blue litmus solution.
The acidic form of litmus solution is red in colour and called red litmus solution.
Blue litmus solution(blue in colour): It is obtained by making the purple litmus extract alkaline. Thus, it is basic in nature and acts as an acid-indicator by giving a characteristic change in its colour in acids.
Red litmus solution(red in colour): It is obtained by making the purple litmus extract acidic. Thus it is acidic in nature and acts as a base-indicator by giving a characteristic change in its colour in bases.
Now questions arise:
- How do they (litmus solutions) act as acid –base indicators?
- How do they change their colours in acids and bases?
- How do they test whether the given substance is acidic or basic?
Experiment to test: Take about 2 - 4 ml of distilled water in two test tubes and add 1-2 drops of blue litmus solution in one test tube and red litmus in another test tube. Now add the sample solution of the substance to be tested in both test tubes (fig.)
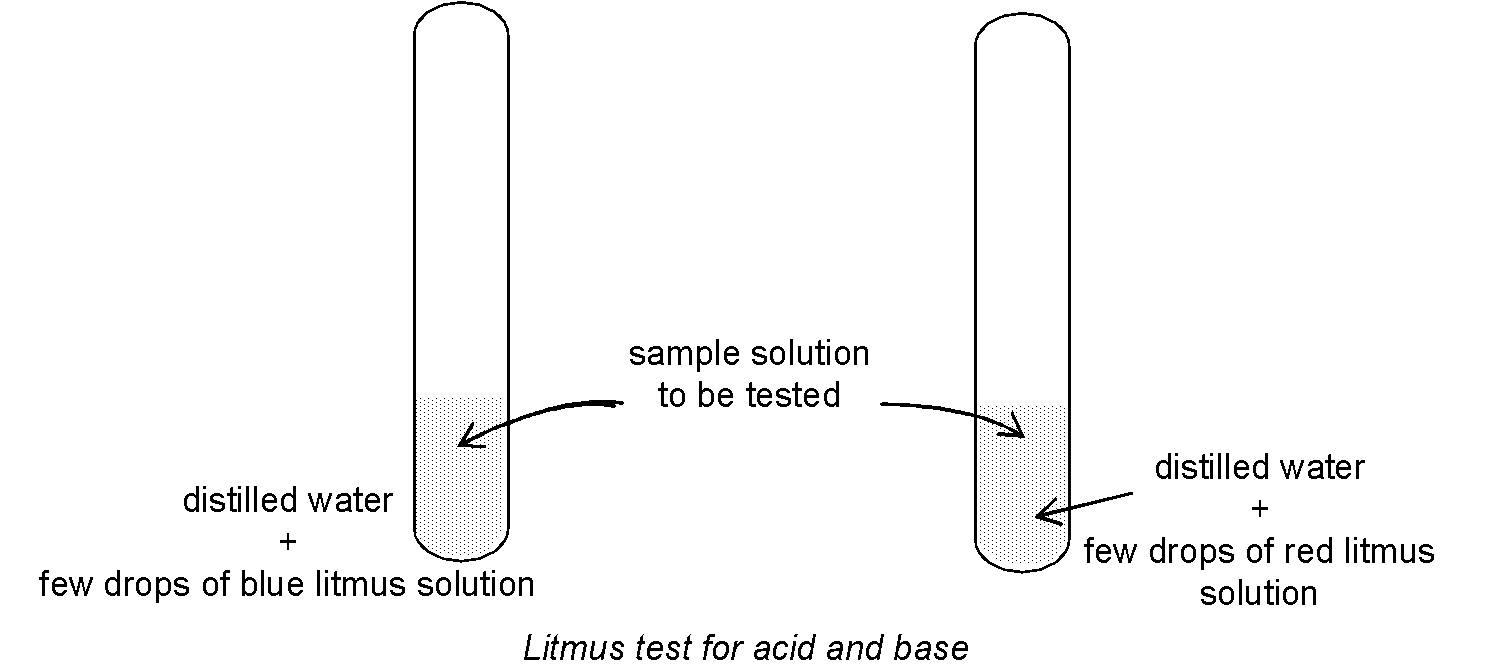
Observation:
- Blue litmus solution turns red in acidic medium i.e. blue litmus solution changes into red if the sample solution (to be tested) is acidic.
- Red litmus solution turns blue in basic medium i.e red litmus solution changes into blue
if the sample solution (to be tested) is basic.
|
|
Change in colour is due to acid or base present in sample solution. |
The above observation can be shown more clearly by taking examples of some commonly used substance as follows.
Table 2
|
Acidic substance turning blue litmus solution into red |
Basic substance turning red litmus solution into blue |
|
Vinegar Lemon Juice Tamarind (imli) Sour milk or curd Proteins Tomatoes Apples Oranges Juice of unripe mangoes |
Baking soda solution Washing soda solution Bitter gourd (karela) extract Cucumber (kheera) extract |
It is clear from the above that blue litmus solution acts as acid indicator by giving red colour in acidic medium and red litmus solution acts as base indicator by giving blue colour in basic medium.
Thus litmus solution acts as an acid-base indicator.
TURMERIC (HALDI):
Turmeric used in kitchen can also be used to test a basic solution i.e. it act as base indicator by giving brown colour in basic medium. In other words yellow colour of haldi turns into brown in basic substances (due to base present in them) and thus distinguishes between acids and bases.
e.g. While eating food, if curry falls on the white clothes, a yellow stain is produced in the clothes. When we apply soap solution (basic in nature) on the cloth, the yellow stain becomes brown due to base present in soap solution.
This example shows that turmeric (haldi) act as base indicator by giving brown colour in basic substances.
SYNTHETIC INDICATORS:
The synthetic chemical substances which change their colour in acids and bases and thus distinguish between them are called synthetic indicators. Since they distinguish between acids and bases, so they are also called synthetic acid base indicators. The two most common synthetic indicators are
(a) Phenolphthalein and
(b) Methyl orange.
Now questions arise
- How do they (synthetic indicators) act as acid-base indicators?
- How do they change their colour in acids and bases?
- How do they test whether the given substance is acidic or basic?
Experiment to test: Take about 2 ml of sample solution (substance to be tested) in two test tubes and add 2-3 drops of phenolphthalein and methyl orange (synthetic acid-base indicators) to then as shown in figure.
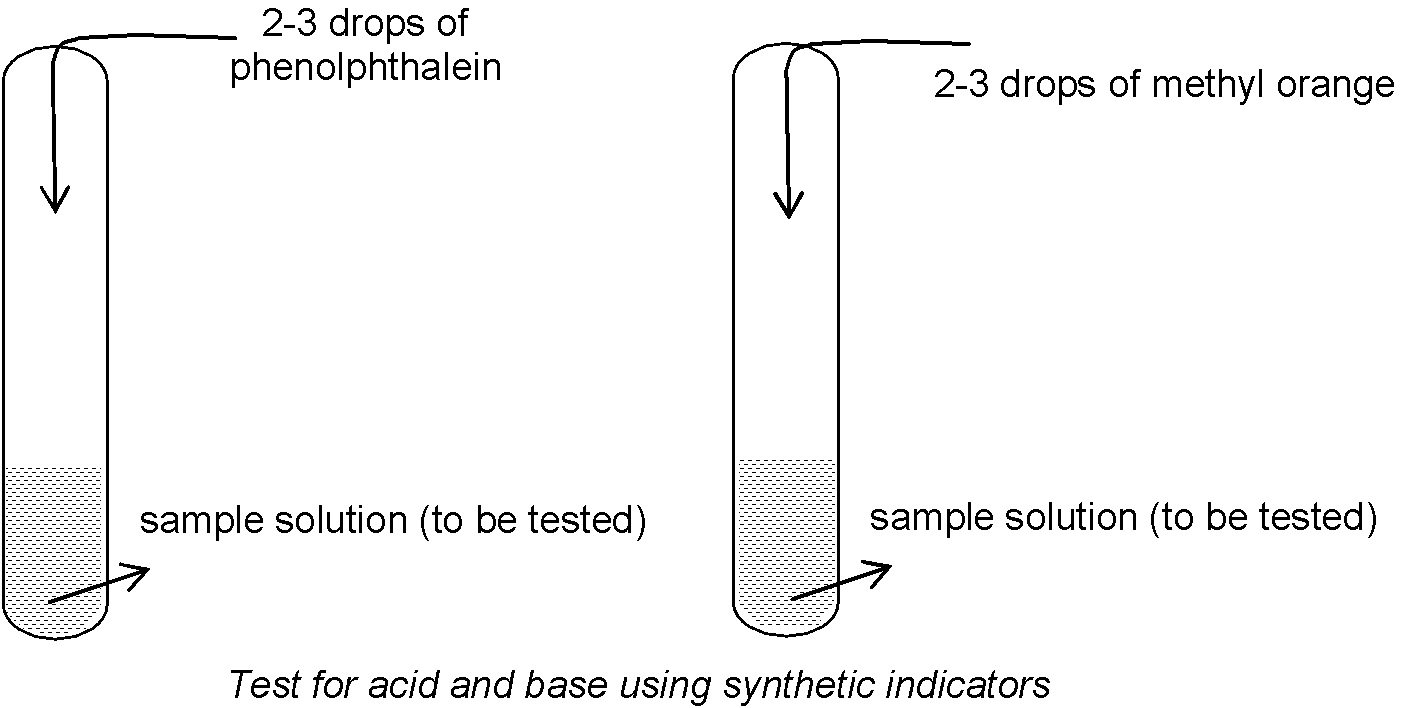
Observation:
(a) Colour changes which take place in phenolphthalein are
(i) Phenolphthalein (whose natural colour is colourless) is colourless in acidic medium.
(ii) Phenolphthalein gives pink colour in basic medium or solution.
(b) Colour changes which take place in methyl orange are
(i) Methyl orange (whose natural colour is orange) gives pink colour in acidic medium or solution.
(ii) Methyl orange gives yellow colour in basic medium or solution.
The above observation can be shown clearly by taking examples of some commonly used substances as follows:
Table - 3
|
Acidic substances turning methyl orange into pink and phenolphthalein remaining colourless |
Basic substances turning methyl orange into yellow and phenolphthalein into pink |
|
Vinegar Lemon Juice Tamarind (imli) Sour milk or curd Proteins Tomatoes Oranges Juice of unripe mangoes |
Baking soda solution Washing soda solution Bitter gourd (karela) extract Cucumber (kheera) extract |
Now, if we see table 2 and table 3 observations, then we conclude that acid – base indicators like (litmus solution i.e. blue litmus solution and red litmus solution), phenolphthalein and methyl orange distinguishes between acids and bases by giving different colours (table 4)
Table – 4
|
Sample solution |
Red litmus solution |
Blue litmus solution |
Phenolphthalein indicator |
Methyl orange indicator |
|
Vinegar |
No colour change |
Red |
Colourless |
Pink |
|
Lemon juice |
No colour change |
Red |
Colourless |
Pink |
|
Washing soda solution |
Blue |
No colour change |
Pink |
Yellow |
|
Baking soda solution |
Blue |
No colour change |
Pink |
Yellow |
|
Tamarind (imli) |
No colour change |
Red |
Colourless |
Pink |
|
Sour milk or curd |
No colour change |
Red |
Colourless |
Pink |
|
Proteins |
No colour change |
Red |
Colourless |
Pink |
|
Bitter gourd (karela) extract |
Blue |
No colour change |
Pink |
Yellow |
|
Oranges |
No colour change |
Red |
Colourless |
Pink |
|
Cucumber (kheera)Extract |
Blue |
No colour change |
Pink |
Yellow |
|
Tomatoes |
No colour change |
Red |
Colourless |
Pink |
|
Juice of unripe mangoes |
No colour change |
Red |
Colourless |
Pink |
The above observation can be shown more clearly as follows
Table – 5
|
Indicator |
Colour in acidic solution |
Colour in basic solution |
|
Blue litmus solution Red litmus solution Phenolphthalein Methyl orange |
Red No colour change Colourless Pink |
No colour change Blue Pink Yellow |
It is clear from the above table that to test whether a substance is acidic or basic we can use any one of the above indicators. The change in colour with these indicators for the substance taken, shows its acidic or basic nature.
NEUTRALISATION:
It may be defined as a reaction between acid and base present in aqueous solution to form salt and water.
HCl (aq) + NaOH (aq) ![]() NaCl (aq) +
H2O (l)
NaCl (aq) +
H2O (l)
Basically neutralisation is the combination between H+ ions of the acid with OH- ions of the base to form H2O.
For e.g. H+(aq) + Cl−(aq) + Na+(aq) + OH−(aq) ![]() Na+(aq) + Cl−(aq) + H2O(l)
Na+(aq) + Cl−(aq) + H2O(l)
H+(aq) + OH−(aq) ![]() H2O(l)
H2O(l)
Neutralisation reaction involving an acid and base is of exothermic nature. Heat is evolved in all neutralisation reactions. If both acid and base are strong, the value of heat energy evolved remains same irrespective of their nature.
For e.g. ![]()
![]()
Strong acids and strong bases are completely ionised of their own in the solution, No energy is needed for their ionisation. Since the cation of base and anion of acid on both sides of the equation cancels out completely, the heat evolved is given by the followir4 reaction
Reaction of strong acid and strong base evolves 57.14 J.APPLICATIONS OF NEUTRALISATION:
People particularly of old age suffer from acidity problems in the stomach which is caused mainly due to release of excessive gastric juices containing HCI. The acidity is neutralised by antacid tablets which contain sodium hydrogen carbonate (baking soda), magnesium hydroxide etc.
The stings of bees and ants contain formic acid. Its corrosive and poisonous effect can be neutralised by rubbing soap which contains NaOH (an alkali).
The stings of wasps contain an alkali and its poisonous effect can be neutralised by an acid like acetic acid (present in vinegar).
Farmers generally neutralise the effect of acidity in the soil caused by acid rain by adding slaked lime (Calcium hydroxide) to the soil.
