Writing A chemical equation
when a magnesium ribbon is burnt in oxygen, it gets converted to magnesium oxide. This is the statement form of a chemical reaction but it can be write as a following manners
Magnesium + Oxygen → magnesium oxide
(Reactants) (Product)
To write the equation in such manner is called as word equation.
Another method to write chemical reaction;
Mg + O2 → MgO
This is symbolic form to write chemical equations.
There are two part of a chemical reaction
- Reactants: The substances that undergo chemical change in the reaction are the reactants. Example from above equation. Mg and O are reactants.
- Procducts: The new substance, formed during the reaction, is the product. Ex. MgO is product, which newer substance produced in reaction.
Writing A chemical equation
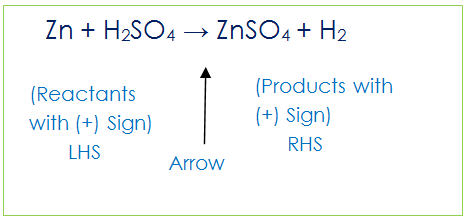
A chemical equation represents a chemical reaction. So reactants are on the left hand side (LHS) of arrow and there is written (+) Sign between them. Similarly, Products are on the Right hand side (RHS) of arrow with a plus (+) sign between them.
Consider this;
Skeletal Chemical Equation
Mg + O2 → MgO
Observe this equation, count and compare the number of atoms of each element on the LHS and RHS of the arrow. There is no equal numbers of atoms of each element on both sides. There is a little unbalance in number of atoms of Oxygen. There are 2 on left and 1 on the right of arrow. Unbalance chemical equation is called skeletal equation. hi